Assembling Version2 of the UCSF500 Cancer Gene Panel
The CCGL has recently validated and launched the UCSF500 cancer panel for patient care. The panel is used on clinical samples, sequencing both tumor and normal tissue to identify the drivers within a patient’s tumor and potential therapeutic targets. To request UCSF500 testing, please fill out our UCSF500 Testing Request form.
We are now designing version 2 of the UCSF500 and are accepting gene requests. As in version 1, the assay uses hybridization-based target enrichment of approximately 500 cancer genes for sequencing and analysis of mutation and copy number changes. Selected genes are also analyzed for structural rearrangements (listed separately below). The assay does not asses epigenetic alterations.
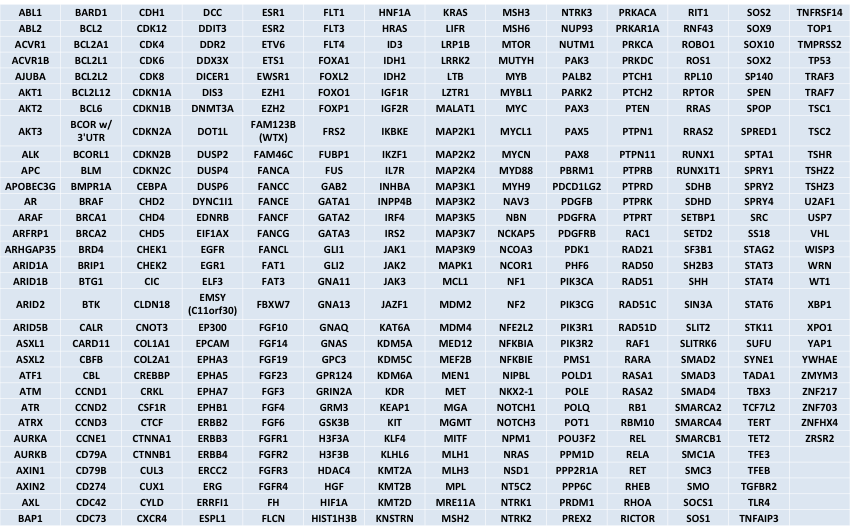
Genes that will be included
All coding regions of these genes will be tiled on version2 of the UCSF500 panel. This will allow for mutation detection (including single nucleotide variants and small insertions/deletions) as well as copy number detection (amplification and deletion) in these genes.
Rearrangements that will be included
Select introns of these genes will be tiled on version2 of the UCSF500 panel for fusion and other rearrangement detection.

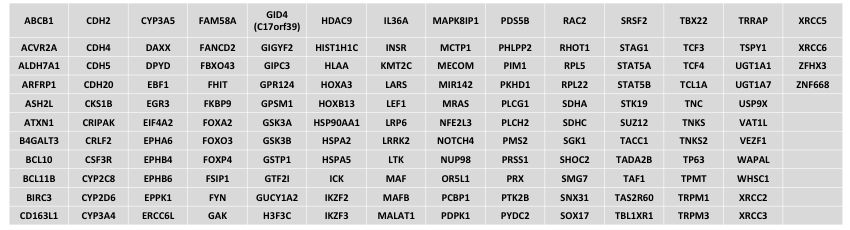
Genes that will be removed
This list contains mostly legacy genes not frequently mutated in cancer, but also includes some cancer relevant genes that cannot be reliably assessed due to technical reasons.
Submit Gene Request
Submission for Version 2 of the UCSF 500 Panel were open until November 13, 2015.