
Clinical research at the HDFCCC is supported by four units:
Clinical Research Support Office (CRSO)
Clinical Research Programs
- Provides central oversight and a core of personnel with expertise in all types of clinical trials management
- Supports investigators in the conduct of cancer-related therapeutic or nontherapeutic clinical trials
Regulatory Affairs
- Provides protocol development and editing, consent form development, full regulatory compliance, and IND/IDE filing and maintenance
- Prepare and submit Institutional Review Board (IRB) applications and ancillary safety committees (i.e. Radiation Safety).
Contact information:
CRSO Medical Director
Nicholas Butowski, MD | [email protected]
CRSO Director
Andrea Skafel, MSc, CCRP | [email protected]
Protocol Review and Monitoring System (PRMS)
- Disease or modality specific scientific and feasibility review
- Prioritization of each new concept and protocol as it relates to the patient population and research focus of each site committee
Site Committee Chairs and Administrators
Protocol Review and Monitoring Committee (PRMC)
- HDFCCC-wide scientific and feasibility review
- Monitors for adequate accrual and ongoing scientific relevance, and closes studies that do not accrue or lose scientific relevance.
Contact information:
PRMC Chair
Matthew Gubens, MD, MS | [email protected]
PRMC Administrator
Jenna Weight | [email protected]
Data and Safety Monitoring Committee (DSMC)
- Monitoring and Auditing of interventional IITs and NCI cooperative group trials
- Preparation of study teams for external audits and inspections
Education and Training Office (MyAccess login required)
- Onboarding and continual education of clinical research staff
- The following training/resources are available:
- CRC Onboarding Series
- Regulatory Training Series
- Investigator Training
- Continual Education
- SoCRA Exam Preparation
NCI Approved DSMP (version 27 Mar 2025)
Abbreviated DSMP Template for Protocols (version 29Aug2025)
DSMP Approval Letter (version 29 April 2025)
DSMC Roster (v 30 Jan 2025)
Contact Information:
DSMC Chair
Katie Kelley, MD | [email protected]
DSMC Director
John F. McAdams, MS, CCRP | [email protected]
Clinical Research Network Office (CRNO)
Regional Affiliate Partnerships
- Develop, streamline and improve oncology clinical research opportunities at partner sites around the bay area
National Clinical Trials Network (NCTN)
- Manage the UCSF NCTN program and all associated affiliate sites
Contact Information:
CRNO Medical Director
Mary Feng, MD | [email protected]
CRNO Director
Arla Yost, MSc, CCRP | [email protected]
These units are guided by the Cancer Center Clinical Research Oversight Committee (CCCROC).
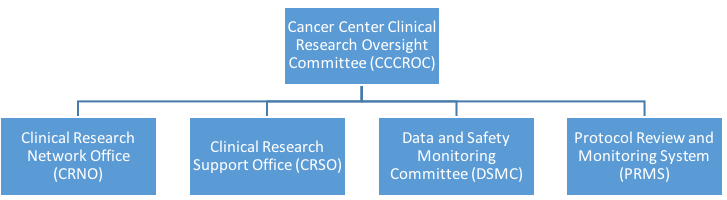
Together the units provide a centralized mechanism to support cancer clinical research and assist HDFCCC members with conducting clinical studies in compliance with all federal, state and local regulatory requirements.
Additional Resources
This unit is supported by a National Cancer Institute Cancer Center Support Grant (P30CA082103). Any publications related to work done by this core should reference grant number P30CA082103 and must include a PMCID as required by the NIH. View instructions on how to acknowledge funding sources and how to obtain a PMCID.
For inquiries about Cancer Center Shared Resources, please email Benjamin Braun, MD, PhD.