Adam Renslo and Michelle Arkin co-lead the Small Molecule Discovery Core at the UCSF Helen Diller Family Comprehensive Cancer Center.
The goal of the Small Molecule Discovery Core (SMD) is to help the Cancer Center and other investigators initiate drug discovery and chemical biology projects for their most innovative and exciting new targets.
Faculty co-directors Adam Renslo, PhD and Michelle Arkin, PhD, both of UCSF’s Pharmaceutical Chemistry Department, took a moment to talk about the role the SMDC plays in accelerating cancer research and precision medicine at UCSF and beyond.
Q: Whom does the HDFCCC Small Molecule Discovery Core serve and how:
MICHELLE: UCSF has great basic scientists and clinicians who understand bio entrepreneurship and how to turn an idea into something that might eventually become a therapeutic. We turn their biology into assays that can be tested many times to find new molecules, and then we characterize how those molecules are working to optimize them.
ADAM: The way that I like to think about what we do is that we make prototype drugs. There’s a new biological pathway, and we ask what would a drug look like that works on this pathway. Maybe it’s too nascent an idea for pharma to get excited about, but can we see how a molecule that works on the pathway behaves in an animal model? If it’s encouraging, hopefully you’ll get a publication or maybe spin out a company. That’s the exit. It’s not getting a drug on market. Rather, it’s accelerating or seeding that process within academia. It is de-risking the new biology for translation for pharmaceuticals.
Friday, Nov 16, 2018
Inaugural Chemical Biology Consortium Symposium: Bridging the Gaps of Drug Discovery
UCSF Mission Bay Conference Center
presented by the UCSF HDFCCC Small Molecule Discovery Core and the NCI NExT Program
ADAM: The core of what industry does is to try to find drugs. They will generally start from biology that is relatively well validated, often working from a target that already has a drug on the market. They are being pragmatic, focusing on things shown to work. What we do is earlier stage with new biology. It’s riskier but can point to truly revolutionary new therapies.
MICHELLE: Unlike pharma, UCSF has access to patient samples that are critical for precision medicine. Also, there is access to clinician-scientists who are working with cancer patients every day. They really see the needs and then go back into their labs and attempt to fix those problems.
We are trying to increase our ability to work with fewer compounds but across a much more complicated biology space by using more patient-derived samples and combination therapies. That way, we’re validating new targets and compounds as well as a therapeutics strategy that utilizes compounds that you wouldn’t necessarily think would work because they were designed for a different disease.
-Michelle Arkin
Q: What is the state of drug development at UCSF?
MICHELLE: Revolutionary things have happened over the last decade at UCSF in target identification and validation. There has also been a revolution in using complex cell models to understand disease in a way that better recapitulates the human disease (and organs that are often the targets of toxicity) in a dish. We can use these models in a high-throughput format to learn how a drug prototype works and how to make it work better on the disease while avoiding healthy tissues.
In the old days of drug discovery, we oversimplified the systems and screened for things that were too far away from the biology we were trying to model. Chemists would make molecules that were bound very tightly but weren’t soluble, or they got chewed up right away by the liver and never made it where they needed to.
In the last ten years, the drug discovery ecosystem has become much more focused on disease-relevant models and on modeling all aspects of the compounds early. The ATOM Consortium (a public-private partnership including UCSF, GSK, NCI, and Lawrence Livermore National Labs) is trying to take advantage of those breakthroughs by modeling disease experimentally and computationally much earlier in drug discovery and in a much more profound way.
ADAM: Being at UCSF, we have benefited from new computational methods developed in just the last ten years or so. We work with Matt Jacobsen to predict permeability of small molecules and whether they might be kicked back out of cells by efflux pumps. These methods enable drug discovery by trying to capture more of the complexity of what a drug has to do. Those in silico models are emerging at UCSF, and we can be the first to give them a dry run.
Q: Is a lack of funding a primary challenge for your work?
MICHELLE: It’s not just the money, it’s the type of money. Our work is funded in a piecemeal way. The amount of money is not adequate to really do drug discovery, which is expensive, takes a long time, and uses many people. There are a numerous infrastructure costs.

Sample equipment, courtesy the School of Pharmacy.
Q: What equipment or service does the Core have that is invaluable to UCSF investigators?
MICHELLE: Early stage drug discovery is an equipment and computationally intensive activity. We have integrated robotics systems and informatics that allow us to link the hundreds of thousands of data points. We use a very nice home-built database and informatics system we call HiTS. HiTS collects all the data and does a lot of the analysis and compares compounds to each other based on structure.
When a project starts, we work with the investigators to help them design an assay that really answers the questions they’re trying to answer, then help collect and analyze the data. Biology and chemistry groups in the SMDC can then work with the collaborating lab to select exciting compounds, identify potential liabilities, and decide what to buy or make next.
We recently received a $500K grant from the Dean’s Strategic Initiative Fund to purchase a high content imager that does confocal 3D microscopy at high throughput. This allows us to evaluate up to 200,000 compounds for how they affect the biological assay in a more complex way. We’re trying to upend the drug discovery process, doing a lot of the heavy lifting in the computational space and then using experiments to optimize those models.
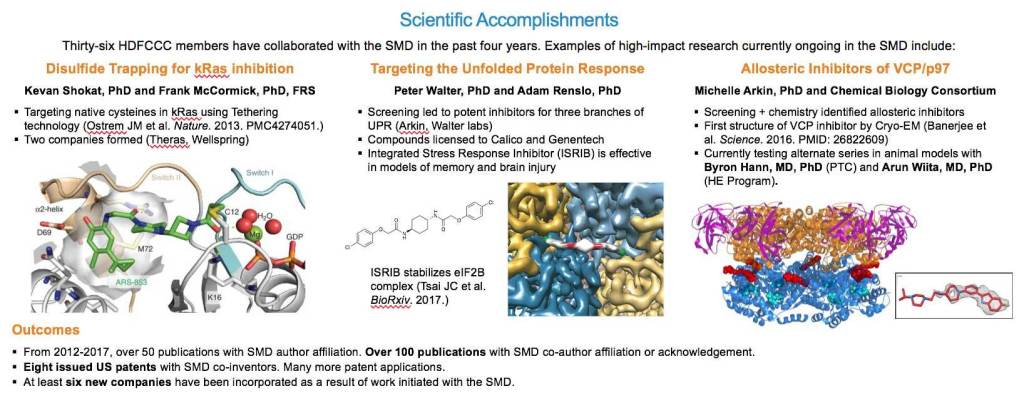
ADAM: Aside from all the cool instruments, we also do a form of screening called disulfide tethering that is fairly unique to our center. It is a different way of finding molecules that relies on the chemical reactivity of cysteines, one of the amino acids present in a protein naturally. You can target an existing cysteine, or introduce a cysteine to target molecules to a specific site on a protein. This is not possible with traditional screening approaches. We’ve built up the infrastructure, the instrumentation, and the chemical libraries to do that special type of screening. And tethering has led to some of our higher profile success stories in cancer.
MICHELLE: The disulfide tethering technology is interesting because it speaks to one of the advantages of our ecosystem. Jim Wells started the Small Molecule Discovery Center and hired us to run it. He founded Sunesis Pharmaceuticals which developed the disulfide tethering technology. Adam’s group has completely rebuilt the library, and we’ve updated the automation and informatics. The results are read out by mass spectrometry, and we have been able to piggy back on that to improve the technology.
Adam Renslo discussed the Core's services with Cancer Center members at a poster session of the recent NCI CCSG renewal.
Q: What high profile projects and collaborations has the Core been involved in?
ADAM: Certainly RAS is one of them. RAS is such a well-validated target in cancer but it has been so difficult to develop drugs for that the field had kind of lost hope. Kevan Shokat and Frank McCormick came to us with the idea of using tethering technology to go after RAS in new ways. Thanks in part to the success of these collaborations, there’s a resurgence of interest and optimism about targeting RAS. We can take some credit for that. And both of those projects led to spin out companies that are working towards drug candidates.
MICHELLE: Another big project is Peter Walter’s Unfolded Protein Response, that started with four high-throughput screens with my team and developed over several years with Adam’s. This project has yielded exciting molecules that have led to biotech collaborations and have been great tools for exploring biology.
ADAM: For instance, Davide Ruggero has shown that one series of compounds we call Integrated Stress Response InhiBitors (ISRIB), can be very effective in especially aggressive prostate cancers that highjack the the stress response pathway. And there is now a cryo-EM structure of the ISRIB compound bound to its target, eIF2B. The structure was solved by Adam Frost’s lab at UCSF and is impressive because eIF2B is this massive protein machine, and that little ISRIB molecule is just sitting right in the middle. This showcases novel small molecule pharmacology - a new example of what a small molecule can do. Finding these first-in-class molecules is what we strive to do.
MICHELLE: My research is in protein-protein interactions and p97 plays a number of important roles in biology governed by different protein complexes that it forms. What makes it a high-profile story from the SMDC perspective is that we (with the NCI CBC) have developed potent allosteric inhibitors of this enzyme. We worked with the NCI to solve the first cryo-EM structures of this protein bound to a small molecule, published in 2016. I think it was only the second publication describing a small molecule bound to a protein solved by cryo-EM at atomic resolution. Parenthetically, the use of cryo-EM for drug discovery is a very exciting, emerging area.
Q: What audience at UCSF would you like to collaborate with more?
ADAM: We would like to meet more investigators at Parnassus and Mount Zion. We have fewer collaborators from those sites than from Mission Bay.
Q: Does the Core have many users outside UCSF?
MICHELLE: Yes, especially from other UCs, biotech companies, and investigators who want to try the disulfide tethering technology. We are also striving to build collaborative, team science. To really advance drug discovery in cancer, you need people with different expertise collaborating.
>More information about the Small Molecular Discovery Core