The Preclinical Therapeutics Core (PTC):
- Conducts preclinical oncology trials for UCSF Helen Diller Family Comprehensive Cancer Center investigators and industry partners.
- Offers an extensive set of in vivo services covered under a comprehensive IACUC protocol, and provides training for common animal procedures and survival surgeries.
- Oversees and provides access and user training for multiple small animal imaging devices housed within the barrier facility.
- Manages and provides user training for various irradiator technologies, including precision irradiation.
- Imaging projects and irradiation experiments can be run entirely by Core personnel, if requested.
Facilities
The Core utilizes two levels for animal hygiene, a standard specific pathogen free (spf) facility for immunocompetent mice, and a super clean facility with additional precautions to prevent infection of immunocompromised mice, such as those used to grow human tumors in vivo.
In experiments where the immune system is not involved, animals are housed in a regular room in the same building (HD525). In here, immunocompetent mice are used to conduct experiments with murine cancer cells.
The Core manages a room dedicated for imaging studies (HD527). Imaging instruments in this room are accessible to all members of the UCSF scientific community.
In vivo Services
Cell and tissue engraftment
- The PTC has expertise in high volume cell culture, and maintains a large cryorepository of commonly used tumor cell lines and tissues.
- Xenografts or allografts are generated via cell and/or tissue implantation; as well as orthotopic engraftment in different mouse strains depending on the required animal immune status. The cells and/or tissue sample can be implanted in different locations based on the desired animal model. In example, subcutaneous, intramammary fat pad, intracranial, intratibial, etc...
Compound formulation and delivery
- The PTC has expertise in formulating experimental agents for delivery using a variety of commonly used methods (i.e. oral gavage, intra-peritoneal, intra-venous (tail vein or retroorbital injections), hydrodynamic, intra-tumoral, subcutaneous and osmotic mini-pumps).
Animal monitoring and tissue collection

- Mice are routinely monitored by body weight and physical observation following the institutional welfare guidelines. Tumor measurement is preformed using digital calipers.
- In vivo imaging is also used to assess tumor burden, location and dissemination. Available imaging technologies includes μCT, ultrasound and optical imaging.
- PTC performs collection of body fluids (including blood, thoracic and peritoneal effusions and urine), tissues and tumors as needed per experimental design. Standard tissue processing includes fixation for paraffin/OCT embedding, snap-freezing, and cryopreservation.
Pharmacokinetic (PK) and pharmacodynamic (PD) analysis
- The Core assists the scientific community in their PD and PK studies under request.
- For PK analysis, after single or multi-day delivery of experimental agent, plasma/serum is collected at multiple time points over a 24-72 hr period for assessment of drug persistency (note: analyte determination is run by user).
- For PD analysis, animals are typically treated with an experimental agent for 3-5 days before tissue/tumor collection for molecular target analysis or biodistribution.
Patient derived tumor xenografting

- The Core engrafts fresh human tumor tissue derived from patient biopsy or surgery into immunodeficient mice in the clean area.
- After tumor growth, tissue is collected by surgical removal, dissected into multiple fragments, and reimplanted into a new batch of host mice. Other fragments are cryopreserved and banked for further work.
Models of metastatic disease
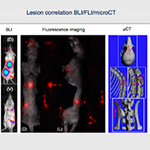
- The PTC generates new and established models of metastatic disease for cancer research. This is done by intravenous injection of cancer cells in most of the times, but intracardiac injection can be performed too, with ultrasound guidance. In most cases, molecular imaging methods allow evaluation of tumor location and overall tumor burden, including metastases.
Surgical procedure training
- The PTC in collaboration with LARC has developed a program of certified training for survival surgery procedures. If required, the Core staff can preform the surgeries for the users in case emergency or lack of trained lab personnel.
- The PTC provides both 1:1 session and hands-on training in addition to assist students and research fellows who need to learn surgical procedures.
Imaging Services
The PTC oversees and provides access and user training for multiple small animal imaging devices housed within the barrier facilities at Mission Bay (HD503 and HD527), Mount Zion (S0171) and Parnassus (PSB554) campuses. If necessary, PTC can run the imaging experiments by request.
Vevo 2100 (HD527) and Vevo 770 (PSB554) High Frequency Ultrasound
- Visualization and quantification of small animal anatomical targets in tandem with hemodynamic evaluation with resolution as low as 30 microns
- Non-invasive, non-radioactive, therapeutic regimes can be monitored in the same animal over time
- Power Doppler mode allows for blood flow quantification & anatomical identification
- 3D Imaging & Volume Analysis
- Contrast enhanced imaging and quantification
- Presence of molecular biomarkers
- Not applicable for brain or lung imaging
Xenogen IVIS Spectrum Fluorescent and Bioluminescent Imager (HD538, PSB554, S071)

- Quantitative imaging of bioluminescent or fluorescent tumor cells/probes within small live animal models
- Multispectral fluorescent imaging options
- Dual illumination capability enables topographic localization of both shallow and deep tumors in 3-D and reduces background interference.
- 20µM Resolution at 3.9cM field width
Small Animal Radiation Research Platform (SARRP) uCT-guided Precision Irradiator (HD-537)

- SARRP delivers targeted radiation to pre-clinical animal models with an accuracy equivalent to clinical radiotherapy
- Using on-board high resolution 3-D cone beam-Computed Tomography (CT) imaging, the SARRP can target micro beams (down to 0.5mm) of radiation achieving an accuracy of 200um for irradiated target
- SARRP software allows user to image animal, contour the tumor/target and at risk organs, evaluate dose, and deliver the desired treatment
Quantum G2X uCT (HD527)
- A clinical device translated into lab animal's size. The scanning bed and technical parameters are adapted to rodents and the system provides anatomical and functional information.
- Ideal for lung and bone studies, but also useful in soft tissue analysis using contrast agents.
- High resolution (2.3 uM voxel size) and High-speed scanning (scans as fast as 3.9 seconds)
- Low-dose imaging for longitudinal in vivo studies, avoiding the potential radiotherapeutic effect on the animals and
- Four Field of Views (FOVs) –18, 36, 72, and 86mm
- Multispecies imaging capabilities (Zebrafish/mouse/rat/guinea pig/rabbit) Two-phase retrospective respiratory and cardiac gating, ideal for imaging heart and lungs Seamless co-registration of functional optical signals (from IVIS® Spectrum or FMT®) with microCT imaging data
Additional Resources
A searchable database of core facilities at all UCSF campus locations is available here.