The Tobacco and Carcinogen Biomarkers Core serves as an analytical chemistry resource for the UCSF tobacco control and cancer research community. The core’s goal is to provide a service for measuring tobacco exposure biomarkers and selected carcinogen biomarkers in biological samples.
The facility’s faculty director is Neal Benowitz, MD, Chief of the Division of Clinical Pharmacology. Core staff include Research Chemist Peyton Jacob, III, PhD, Laboratory Director, and Lisa Yu, BS, Laboratory Manager.
Services
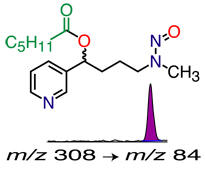 illustrated: analysis for the tobacco-specific carcinogen biomarker NNAL |
The Clinical Pharmacology Laboratory (CPL) at UCSF/SFGH provides a service for analysis of biological samples for tobacco biomarkers. These include nicotine and its metabolites and biomarkers for carcinogen exposure. The CPL has analyzed biological samples in support of clinical research studies of tobacco pharmacology and toxicology for more than 30 years. The laboratory has the capability to analyze large numbers of samples and typically analyzes about 10,000 samples per year. The methods are chromatographic and mass spectrometric (GC, GC-MS, GC-MS/MS, and LC-MS/MS), all of which have been developed at the CPL and some of which are not available elsewhere.
Applications Include:
- Validation of smoking cessation or cessation of smokeless tobacco use.
- Measurement of extent of tobacco use (light vs. heavy smoking).
- Identifying and quantifying tobacco use in experimental subjects or patients for possible interactions with therapeutic drugs.
- Phenotyping CYP2A6 activity (using nicotine metabolite ratio), which is highly correlated with the rate of nicotine elimination, and can be useful for individualization of pharmacotherapies for tobacco addiction.
- Studies of secondhand smoke (SHS or ETS) exposure.
- Measuring exposure to tobacco-specific carcinogens—NNAL
- Measuring exposure to polycyclic aromatic hydrocarbons (PAHs), which are a major class of carcinogens in tobacco smoke, that are also derived from other sources such as diet, air pollution, and occupational exposure.
Specific Methods Offered:
GC Cotinine (low sensitivity), for assessing level of tobacco use or validating cessation. Also measures nicotine. Routinely applied to plasma, serum, urine, or saliva.
LC-MS/MS Cotinine (high sensitivity), for assessing exposure to secondhand smoke (SHS). Also measures trans-3’-hydroxycotinine, and can be used to phenotype for CYP2A6 activity by the metabolite ratio. See references in appendix. Routinely measured in plasma, serum, urine, and saliva.
NNAL (4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanol), a tobacco-specific carcinogen metabolite. (LC-MS/MS). Can be measured in urine of smokers or of non-smokers exposed to secondhand smoke (SHS). This is probably the best biomarker for SHS exposure, due to its long biological half-life, by providing a time-averaged measure of exposure when exposure patterns are erratic.
PAH (Polycyclic Aromatic Hydrocarbon) Metabolites. An LC-MS/MS method measures urine concentrations of phenolic metabolites of naphthalene, fluorene, phenanthrene, and pyrene. See references in appendix. PAHs are ubiquitous carcinogens formed during incomplete combustion of organic materials, including tobacco.
Anabasine/Anatabine , tobacco alkaloids, biomarkers for tobacco use during nicotine replacement therapy (NRT). An LC-MS/MS method is used for urine specimens. For validating tobacco use cessation or for estimating extent of tobacco use, the nicotine metabolite cotinine is widely applicable. However, it cannot be used in patients or experimental subjects who are being treated with nicotine-containing medications (patches, gum, or nasal spray) because the nicotine metabolite cotinine is produced from the medication as well as from tobacco. These two tobacco alkaloids are not present in NRT medications and can be used to validate cessation during NRT.
Tissue Digestion. Biological samples that are not fluid, for example biopsy samples, animal tissue, hair, or nails can be digested and analyzed by above methods for an additional fee.
Equipment
 shown: Thermo-Finnigan TSQ Quantum Ultra mass spectrometer |
The CPL currently has four mass spectrometers, including three triple quadrupole systems and a desktop GC-MS system. Two gas chromatographs are also used for measuring smokers’ levels of nicotine and its metabolite cotinine. The instruments are: An LC-MS/MS system consisting of a ThermoFinnigan TSQ Quantum Ultra triple-stage quadrupole mass spectrometer with atmospheric pressure chemical ionization (APCI) and electrospray ionization (ESI) ion sources, which is coupled to an Agilent 1200 liquid chromatograph; a Varian GC-MS/MS system consisting of a 450 gas chromatograph interfaced with a 320 triple quadrupole mass spectrometer with electron ionization (EI) and positive/negative chemical ionization (CI) sources; a an LC-MS/MS system consisting of a ThermoFinnigan TSQ 7000 triple-stage quadrupole mass spectrometer (APCI and ESI sources) coupled with a Hewlett-Packard 1090 liquid chromatograph; an Agilent desktop GC-MS system consisting of a 6890 GC and 5973 mass selective detector (EI and CI sources); a Hewlett-Packard 5890 and an Agilent 6890 gas chromatograph with nitrogen-phosphorus and flame ionization detectors.
Additional Resources
A searchable database of core facilities at all UCSF campus locations, provided by the Clinical and Translational Science Institute at UCSF, is available here.