
UCSF Physician-scientist Mark Moasser, MD, recently published two studies in CELL REPORTS that resolve a longstanding mystery surrounding resiliency in the HER2 protein, a critical driver of the protein HER2, a very important driver of breast cancers and many other types of cancer.
One of the major successes of decades of cancer research has been the development of drugs that specifically inactivate oncogenes, genes that function abnormally, causing cells to behave erratically, become malignant and make tumors. These drugs are highly effective treatments for cancers caused by oncogenes.
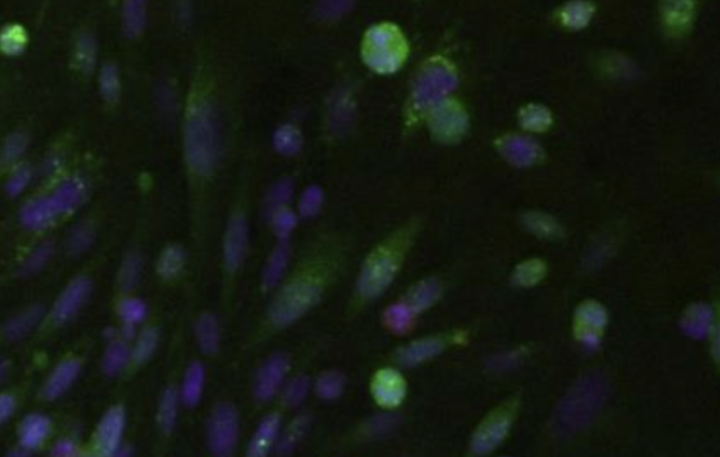
Microscopic image showing parts of the HER2 receptor touching each other inside the cells (green) and outside the cells (red) in a process called dimerization. The lack of red shows that cells with high levels of HER2 can have a lot of dimerization of their inside halves, which helps sustain cancer, without any dimerization of their outside halves. Image by UCSF
Monoclonal antibodies are one of the main classes of pharmaceuticals, and a mainstay in the cancer therapeutic arena. Such drugs can bind to a specific target protein and halt its functioning. Small molecule drugs, another mainstay of cancer therapeutics, are synthetic drugs that can be designed to fit into certain pockets in proteins and stop them from functioning.
However, success with these types of treatments has proven elusive for the protein HER2, a very important driver of breast cancers and many other types of cancer. Antibody drugs such as Herceptin or Perjeta were made to inactivate HER2, but they seem to be very weak at doing so. Many others have tried to develop antibodies that inactivate HER2, but without success. Many small molecule drugs have also been made to inactivate HER2, but they also have only weak effects.
Mark Moasser, MD, a physician-scientist at the UCSF Helen Diller Family Comprehensive Cancer Center, recently published two studies in CELL REPORTS that finally resolve the mystery surrounding this resiliency in HER2 and suggest promising directions for much more effective drug development going forward.
What makes HER2 such an important gene to target for cancer treatment?
HER2 is one of the most common oncogenes in human cancers, accounting for about 20 percent of breast cancers, 15 percent of gastric esophageal cancer and smaller percentages of many other types of cancer. In healthy cells, HER2 helps control cell growth and division and it plays a central role in the biology and behavior of many human cancers.
It is also one of the earliest oncogenes discovered in the 1980s and there has been decades of research on it and a lot of investment by the pharmaceutical industry. Indeed, HER2 has been one of the most pursued targets for drug development in cancer therapeutics history.
What did you discover about how HER2 behaves that makes it so challenging?
We know about a lot of oncogenes now. Most of these are activated through mutations in cancer cells, and many drugs have been developed to inactivate these oncogenes with tremendous success. For example, many types of lung cancers are treated highly effectively these days by drugs that inactivate their mutated oncogenes, and these drugs have replaced chemotherapy.